Week 2: drift and neutral dynamics
Before we start talking about ecological drift and the unified neutral theory of biogeography, let’s talk about microevolution. What’s Hardy-Weinberg Equilibrium? Why might there be a deviation from it in a population over time?
The answers to that question are essentially the processes that drive evolution:
Selection
Gene flow
Mutation
Drift
Microevolution (the change in allele frequencies in a population over time) is driven by some combination of those processes. Some people teach HWE as the null hypothesis for allele frequencies in a population over time. But over long enough time stretches, there will still be mutation and drift in the absence of gene flow and selection. So if you want to attribute a change in allele frequencies in a population over time, you still have to account for mutation (especially for large, quickly reproducing populations) and genetic drift (especially for small populations).
Genetic drift arrises from imperfect sampling of one generation to the next. In the absence of natural selection, all organisms in a population make equal contributions to the next generation… but all organisms in a population making exactly equal contributions to the next generation is almost impossible. (See the course glossary for more notes on this)
In very small populations, genetic drift is obvious within a generation or two. In very large populations it’s just slower.
A way we can think about selection is due to Ernst Mayr’s interpretation of Darwin’s 1859 essay. Natural selection will occur if:
1) There is variation within a population
2) Some of that variation is heritable
3) There is variation in the survival and/or reproductive output among individuals in a population
4) #3 is causally connected to #1 in a particular environment.
Sexual selection is basically the same, but #4 is driven by some sort of intra-specific dynamic, often involving some form of mate choice or reproductive compatibility. Artificial selection is when humans are the causal factor linking #1 and #3. Genetic drift is essentially the first three conditions being met without #4.
The reason I like that formulation is that it underscores the essential trickiness of establishing whether the change in allele frequency in a very small (or sometimes even a medium) population is due to selection or drift. Experimentally, researchers approach this epistemological difficulty by “replaying the tape of life,” i.e., by doing replicated selection experiments. If you don't account for drift in your “null” expectations of what allele frequency change looks like through time, you might misinterpret its signal as selection.
In their recent text on evolutionary biology, Futuyma and Kirkpatrick (2017) enumerate some features of genetic drift (p168):
"Drift is unbiased," so the direction of drift one generation is not predictive of the direction of drift in the next generation. This is a general feature of random walks.
“Random fluctuations in allele frequency are larger in smaller populations.”
"Drift causes genetic variation to be lost." The same is true for selection. The fixation of an allele can be caused by drift, or selection. Gene flow and mutation tend to add new alleles.
"Drift causes populations that are initially identical to become different." This property requires replicated experiments to study. It becomes important in understanding Kimura’s Neutral Theory.
"An allele can become fixed without the benefit of natural selection." Hmmm... this seems very similar to the third point... maybe they're just including this for emphasis? At any rate, the loss of alleles due to drift in small populations is analogous to the idea of stochastic extinction on islands (or habitat patches) with low overall populations of organisms (Wright & Hubbell 1983; Simberloff & Abele 1976) due to stochastic extinction.
In evolutionary biology, one of the first suggestions that chance might play a role in evolution was formalized as the Wright-Fisher model of genetic drift in the 1920s and 30s (for more on this history and philosophy of this incarnation of genetic drift, check Plutynski 2007). Although they were not yet sure which biological molecules genes were, Wright intuited that it was unlikely that every heritable unit was under strong selection at all times, writing, for example: “it seems to me that here also those genes which are not controlled by moderately strong selection would ordinarily drift at random through the multiple dimensional system of gene frequencies, regardless of any second or third order evolutionary pressure” (Wright 1929).
Wright was the first to suggest that genetic drift could lead to the accumulation of deleterious alleles in very small populations, and today genetic drift is often invoked for its capacity to effect maladaptive evolution via the fixation of deleterious alleles (Ellstrand and Elam 1993, Kuo et al. 2009) and/or the loss of genetic diversity, which can have long-term consequences for a population’s ability to evolve to new stressors (Lande 1988). Implicit in genetic drift is the possibility that alleles may be neutral or nearly neutral (and that differences we observe among populations might not be driven by adaptive evolution), so, let us introduce the neutral theory of molecular evolution.
As researchers began to generate the first DNA sequences and decipher the genetic code they discovered many synonymous polymorphisms present in populations, which were not explainable by adaptive evolution (Kimura 1968, 1977, 1983). Most obviously, these neutral changes might be at the third position in a codon, which often has at least one mutation it can make to still code for the same amino acid. Similarly, depending on the protein, not every change in amino acid will result in a change in the properties of the protein the gene codes for. Kimura proposed that neutral mutations accumulate in genomes at a roughly constant rate from generation to generation, and that interspecific differences and intra-specific differences in genomes were largely due to genetic drift. This was a radical proposal at a time when genetic differences were thought to be driven by selection.
Kimura’s theory still forms the underpinning of our ability to track changes in effective population size, trace (and potentially time) organismal ancestry with molecular phylogenetics, and other crucial parts of evolutionary biology and conservation genetics (Yoder et al. 2018). However, as our understanding of the way chromosomes work improved, it also became clear that some synonymous mutations could be carried along with adaptive substitutions (Smith and Haigh 1974), and it has become clear that some of the predictions of the original neutral theory have not born out (Hahn 2008, Kern & Hahn 2018). Today, our understanding of the way molecular evolution proceeds is the “weak” version of neutral theory, in which most changes in DNA sequences, particularly those that don’t code for changes in amino acid sequences are lost or fixed due to drift. The “strong” version of neutral theory, in which even nonsynonymous substitutions are neutral, and there is no linkage, is not a useful or parsimonious way of looking at molecular evolution.
An analogous development in ecology is Hubbell’s neutral theory of biodiversity and biogeography. In a review from 2002, Levin’s wrote “...Hubbell suggests a general theory of biodiversity that makes the boldest case yet for regional control over community structure. The theory is radical in that it explains the most essential patterns of community structure through "ecological drift" rather than through interspecific differences in per capita birth, death, dispersal, and competitive rates. In essence, this theory ignores what most ecologists spend their careers studying.”
In Hubbell’s framework, the individuals in an assemblage are assumed to not differ appreciably in their traits (except for abundance and geographic range, which emerge from or are specified in the model), and presence or absence is driven by random dispersal, speciation, and (stochastic) extirpation. Hubbell’s neutral theory builds on the assumptions of MacArthur and Wilson’s theory of island biogeography (MacArthur and Wilson 1963, Wilson and MacArthur 1967), in which the species richness of islands is a function of area, colonization rate, and extinction rate, and species are treated as neutral. In Vellend’s framework we talked about last week, Hubbell’s neutral theory incorporates the processes of speciation, dispersal, and drift, but not selection.
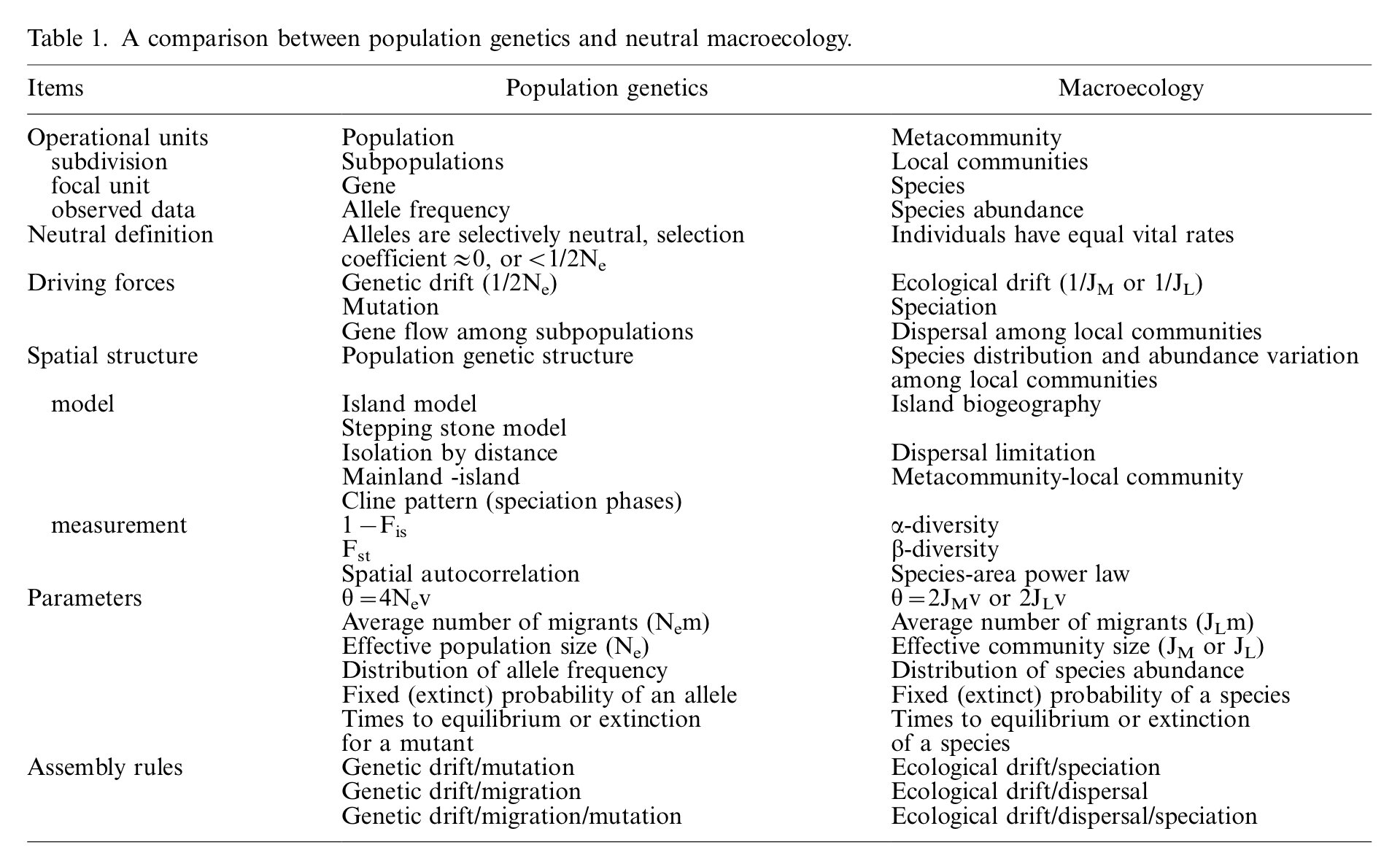
We’re not going to get into the math of genetic drift, but in many cases fairly direct analogues can be drawn between abundance fluctuations of alleles in a population and species abundance in a “neutral” community. Hu et al. 2006 summarize the parallels between genetic drift in population genetics, and Hubbell’s neutral theory in their table 1:
Table 1 from Hu et al. 2006. “Neutral theory in macroecology and population genetics” OIKOS 113:3 548 - 556
Above, what Levin refers to as “…what most ecologists spend their careers studying,” are species differences, and the way differences in species traits and interactions with their environments determine where you find species. In Roughgarden’s work on the philosophy of community ecology, she writes of the niche-assembly driven approaches of the 1970s: “The idea behind niche theory is that interspecific competition causes species to persist in different niches, resulting in a bookcase metaphor for community structure. All communities were envisioned as books stored on a shelf of given length. The books were allowed to overlap a bit on the shelves (niche overlap) and to have varying thicknesses (niche width). A community’s diversity then emerged as the number of books that can be shelved on the book case. The impact on diversity of say, global change or other policy actions could then be predicted by assaying how such actions affected the bookcase length, book width and allowable book overlap and then computing the new diversity that results."
In contrast, an extreme version of community assembly that depends only on niche and nothing else is Baas Becking’s pithy formulation of microbial community assembly: “everything is everywhere; but the environment selects” (see de Wit & Bouvier 2006 ; O’Malley 2007 for perspectives). For organisms with very large populations that are capable of undergoing long distance dispersal and dormancy, this may be close to true at a global scale. For most types of organisms, there are some spatial and temporal extents across which this is true: grasses will colonize a newly vacant lot fairly quickly; and many dragonflies will indiscriminately lay their eggs in whatever body of water they fly by, so that after a year of availability, there are often grasses and dragonfly nymphs in their respective suitable habitats. Dispersal limitation doesn’t strongly constrain grasses and dragonflies at scales of a few miles or less: it’s just a matter of whether the habitat is compatible. At larger spatial scales, there are habitats that may be perfectly suited for a particular species that have never been colonized because it is too far. If “Everything is everywhere; but the environment selects” applied to vertebrates, cats, rabbits, and cane toads would all be native to Australia. [Side note: For macroscopic organisms, the spatially autocorrelated violation of Baas Becking’s perfect niche assembly proposition is Buffon’s law, named after an 18th century naturalist who observed that very similar environments that were far apart usually had different types of plants and animals (see glossary in Lomolino 2016).]
Hubbell’s theory is the opposite. Stochastic dispersal, population fluctuations, and speciation are the only processes governing species distributions. Hubbell was aware in 2001 that seemingly neutral patterns could arise from actual differences among species (p325): “…niche differentiation along life history trade-offs is the very mechanism by which per capita relative fitnesses are equalized among the coexisting species in a community.” (Hubbell, after all, was not ignorant of the differences among species and environments (he did not, for example, suggest that there should be about the same tree assemblage at the bottom of Lago Gatún as there was on BCI). For our purposes, neutral theory will often be useful as a null hypothesis, and potentially a realistic explanation for observed patterns at certain spatial scales. Questions the theory enable us to ask are much more often in the form of “To what extent…” rather than, “is every species the same?” As Hubbell writes (p319 - 320): “The appropriate questions therefore is not whether ecological drift exists, but under what circumstances is it quantitatively important.”
Interestingly, there are some cases when species do seem to be pretty much ecologically equivalent, but Hubbell’s theory is not the perfect fit for understanding their dynamics. Sometimes this happens when ecologically equivalent species occur only (or primarily) in allopatry (as alluded to in Gittenberger 1991). Siepielski & McPeek (2010) explored the ways in which niche theory might not always be the most parsimonious starting assumption, and McPeek & Siepielski (2019) explain how ecologically very similar species might not always exhibit dynamics consistent with Hubbell’s formulation.
Papers we read
Adler, P.B., Hillerislambers, J., & Levine, J.M. (2007) A niche for neutrality. Ecology letters, 10, 95–104.
Chen, B., Shi, Z., Chen, Q., Shen, X., Shibata, D., Wen, H., & Wu, C.-I. (2019) Tumorigenesis as the Paradigm of Quasi-neutral Molecular Evolution. Molecular biology and evolution, 36, 1430–1441.
Jordan, S.M.R., Barraclough, T.G., & Rosindell, J. (2016) Quantifying the effects of the break up of Pangaea on global terrestrial diversification with neutral theory. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 371, 20150221.
Kern, A.D. & Hahn, M.W. (2018) The Neutral Theory in Light of Natural Selection. Molecular biology and evolution, 35, 1366–1371.
Kraft, N.J.B., Cornwell, W.K., Webb, C.O., & Ackerly, D.D. (2007) Trait evolution, community assembly, and the phylogenetic structure of ecological communities. The American naturalist, 170, 271–283.
Overcast, I., Emerson, B.C., & Hickerson, M.J. (2019) An integrated model of population genetics and community ecology. Journal of Biogeography, 46, 816–829.
Rapacciuolo, G. & Blois, J.L. (2019) Understanding ecological change across large spatial, temporal and taxonomic scales: integrating data and methods in light of theory. Ecography, 42, 1247-1266.
Rosindell, J., Hubbell, S.P., He, F., Harmon, L.J., & Etienne, R.S. (2012) The case for ecological neutral theory. Trends in ecology & evolution, 27, 203–208.
Suzuki, T.M. & Chiba, S. (2016) Dynamics of evolutionary radiation under ecological neutrality. Journal of theoretical biology, 406, 1–7.
Vellend, M. (2010) Conceptual synthesis in community ecology. The Quarterly review of biology, 85, 183–206.
Yoder, A.D., Poelstra, J.W., Tiley, G.P., & Williams, R.C. (2018) Neutral Theory Is the Foundation of Conservation Genetics. Molecular biology and evolution, 35, 1322–1326.
Zhou, J. & Ning, D. (2017) Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiology and molecular biology reviews: MMBR, 81, e00002-17.